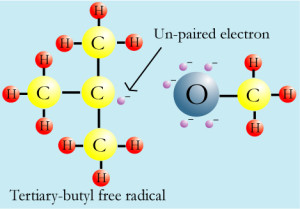
An unpaired electron is an electron that occupies an orbital of an atom by itself, rather than as part of an electron pair. Unpaired electrons are relatively uncommon because an entity that carries an unpaired electron is usually rather reactive. A formation of electron pairs is usually energetically favourable, either in the form of a chemical bond or as a lone pair. In organic chemistry, unpaired electrons typically only occur briefly during a reaction on an entity called a radical. They do, however, play an important role in explaining reaction pathways.
EPR
An EPR, or Electron Paramagnetic Resonance, is a spectroscopic technique that detects species that have unpaired electrons. It is also called ESR (Electron Spin Resonance). Unpaired electrons include free radicals, many transition metal ions and defects in materials.
Free Radical
A radical is an atom, molecule or ion that has unpaired valence electrons or an open electron shell, and therefore may be seen as having one or more “dangling” covalent bonds.
These “dangling” bonds, with some exceptions, make free radicals very chemically reactive toward other substances, or even toward themselves. These molecules will often spontaneously dimerize or polymerize if they come in contact with each other. Most radicals are reasonably stable only at very low concentrations in inert media or in a vacuum.
Paramagnetism
Paramagnetism is a form of magnetism where certain materials are attracted by an externally applied magnetic field.
Paramagnetic properties are due to the presence of some unpaired electrons, and from the realignment of the electron paths caused by the external magnetic field. Paramagnetic materials include magnesium, molybdenum, lithium and tantalum.
The most stable examples of unpaired electrons are found on the atoms and ions of lanthanides. The incomplete f-shell of these entities does not interact very strongly with the environment they are in and this prevents them from being paired. The ion with the largest number of unpaired electrons is Gd3+ (Gadolinium) with seven unpaired electrons.
Related articles:
Scientists find a solution to long-standing mysteries of cuprate high-temperature superconductivity