U.S. Food and Drug Administration (FDA) issued a class one recall of batteries used in a heart-assist device. Everyday Health confirms this reflects their highest level of concern. That’s because the Medtronic heartware ventricular assist device system supports weak blood flow leaving the left ventricle of the heart. The FDA class one recall of faulty heart batteries is therefore a serious matter for this class of heart patients.
The FDA Class One Recall of Faulty Heart Batteries
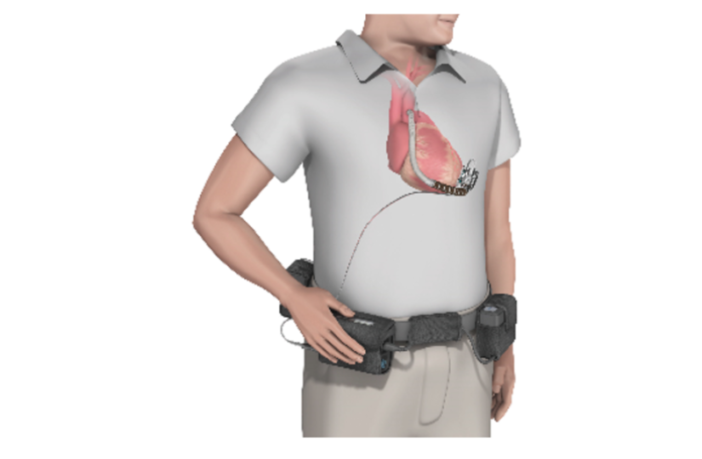
The left ventricle of the heart pumps blood into the aorta, which then distributes the oxygenated fluid throughout the body. The Medtronic heartware ventricular assist device system supports this process, if the natural system is under performing. It is an implant device, with a cable to a compact external controller and battery pack that clips to the user’s belt.
In this way the external batteries allow the user to regain their normal mobility, and continue much as they did before their condition developed. But first, here are details of the product under recall according to U.S. Food and Drug Administration:
- Product Name: Medtronic HVAD Batteries
- Model Numbers: 1650DE
- Devices Being Recalled: 23,372
- Dates distributed: January 1, 2009 to present
- Date Initiated by Firm: June 28, 2022
This Affects Me: What Should I Do Now?
Medtronic is recalling the batteries because they unexpectedly fail. When this occurs, they may be unable to power the controller, unable to accept charge from the battery charger, or appear to remain charged when in use.
Users should therefore do the following while they wait further advice:
- Always have fully charged spare batteries with you at all times.
- Ensure your device is always connected to two power sources.
- Re-read the operating manual to ensure you are using it correctly.
- The battery indicator lights also provide important clues:
… They MUST progressively decrease as the battery runs down.
… If the indicator lights DON’T light up, then the battery is faulty.
… The charger MUST flash red or yellow after disconnecting the battery.
Please see the link below for the full U.S. Food and Drug Administration notice.
Breaking News
Coal Mining Waste to Sustainable Batteries
New Battery Design from Abundant Materials