Scientists at Penn Engineering have taken up the challenge of lithium-ion’s self-destructive personality. They recognize the massive contribution it has made. However, they don’t like the way the electrodes expand and crack with each cycle, meaning they cannot recharge indefinitely. Why not invent self-healing batteries instead, they wondered. This would put less strain on resources.
New Metal Ions Lead to Self-Healing Batteries
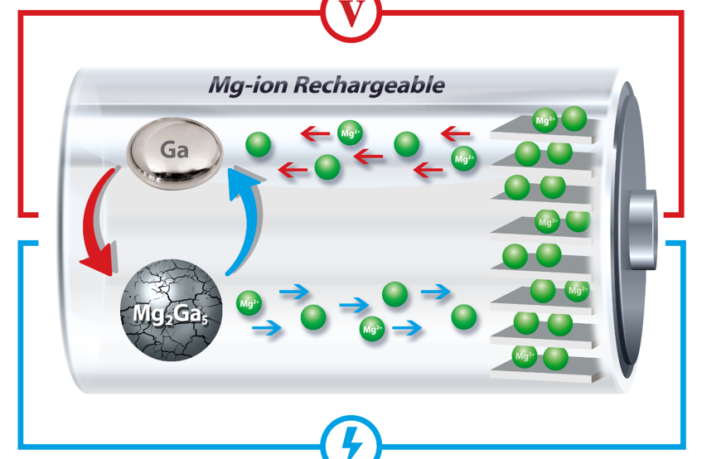
The Penn engineers decided to move on from lithium metal, because of its self-destructive characteristics. They first thought magnesium-ion could be a solution. However, they discovered known compounds of it had even greater cracking and other problems than lithium derivatives. Then they turned their attention to gallium metal.
Gallium is a transition metal in a group of chemical elements that have valence electrons. This enables their electrons to participate in the formation of chemical bonds in two shells, instead of only one. We thought we’d mention it in case you wondered. However, the main point is when they incorporated gallium into a magnesium-ion anode, they opened the door to self-healing batteries.
How Gallium Achieved This in Practice
Gallium has a melting point just slightly above room temperature. This heals the cracking, and the expansion that follows by melting and solidifying after each recycle. The Penn engineers say this extends magnesium-ion battery life such an extent we can speak of them being self-healing batteries.
Moreover, their technology achieves this without using expensive, nano-scale materials. Instead, they alloyed micron-sized particles of gallium with magnesium. We assembled them in a conductive network in order to electronically connect the small pieces, they explain. Our model consists of graphene, carbon fibers, and carbon black which we tied together with a binder.
The gallium does not move around as it goes from solid to liquid as you might expect a liquid to do. That’s because we bundled it in a network of other materials.
Related
Magnesium Ions Dance Through Solid Electrolyte
Magnesium-Ion Batteries: One Step Up From The Li-Ion Technology
Preview Image: Gallium Repeatedly Melts and Solidifies
Video Share Link: https://youtu.be/9DEjE8jiwT8