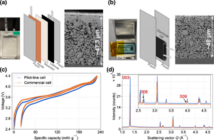
Lithium-ion batteries (LIB) fires are becoming an increasing problem. According to news reports, planes from Boeing Dreamliner and electric cars from Tesla have batteries that burst into flames when exposed to high temperatures (e.g. subjected to overcharging). This endangers not just the passengers but also other people because of the perils of a plane or car crash.
One should note that all vehicles – whether electric or conventional (fossil-fuel-guzzling) – can be hazardous. There is no evidence to suggest that Tesla cars are any more risk-prone than others (there may be evidence to the contrary, in fact), but the relatively new company comes under much scrutiny quite often. This scrutiny is clearly motivated by big-oil interests, since Tesla produces exclusively electric vehicles. And while independence from fossil-fuel use seems inconceivable to most Americans, one hopes for a more environmentally friendly and peaceful future, and so all environmentally friendly enterprises are to be encouraged. (You can read more about renewable power sources).
Electrolytes allow lithium ions to transfer from one electrode to the other while the battery is being charged. When overcharged, these electrolytes can catch fire. These LIBs are also used in mobile devices like smartphones and tablets, but they don’t pose a risk because they are smaller and replaced faster than the ones used in Boeings and Tesla cars.
Scientists have tried to develop a new type of LIB electrolyte that will not make it a fire hazard and still be as effective in delivering stored energy.
A Science Daily article that was published in May 2014, reports that scientists from Cornell University made a discovery that will take us one step closer to solving the flammable electrolyte problem in LIBs. Headed by Lynden Archer and Geoffrey Coates, the team from Cornell University are developing electrolytes that are more stable and solid. While Coates dealt with the problem of solid electrolytes hindering the performance of lithium ions, Archer focused on the electrochemical characteristics of the materials they are using. Their combined efforts resulted in a new type of solid polymer electrolytes that is a cross-link between polyethylene/polyethylene oxide. It is expected to allow the conduction of lithium ions at room temperature while minimizing the fire hazard. The new and improved lithium ion batteries can be used in high-energy lithium-metal batteries (e.g. lithium-sulfur and lithium-air batteries).
In an earlier publication on the same site, Joseph DeSimone of the University of North Carolina (Chapel Hill) identified a possible replacement for the flammable electrolyte in LIBs. He explained that his work sought to use perfluoropolyether (PFPE) as the main ingredient in electrolytes. His team discovered that lithium salt can be dissolved in PFPE and that it exhibits a quality known as ion transport, which makes it a better ingredient to make electrolytes non-flammable without compromising the performance of lithium ions.
These developments will help restore the confidence of people in the use of lithium-ion batteries.
Related Articles:
Infographic: Lithium-Ion Battery
What is a Lithium-ion polymer battery?
Why is Heat Bad for Batteries?
Charging at High and Low Temperature
Scientists find a solution to long-standing mysteries of cuprate high-temperature superconductivity