We must keep our spirits high despite the seemingly slow pace of COVID-19 vaccine development. That’s because the chances of us having a few to choose from by the end of 2020 are high. Moreover, this is incredible progress considering our scientists began their work less than a year ago. Now the AstraZeneca two antibody vaccine is ready for testing too.
What the First AstraZeneca Two Antibody Trial Tests
The U.S. National Library of Medicine project scope (see below) is as follows:
‘In this first-in-humans dose escalation study, AZD7442 (AZD8895 + AZD1061) will be evaluated for safety, tolerability, and pharmacokinetics. And for generation of anti-drug antibodies (ADAs) too. The study is intended to enable future studies of AZD7442’s efficacy in preventing and treating COVID-19’.
The AstraZeneca two antibody vaccine will further test healthy adults with multiple intramuscular and intravenous doses of the drug. And then compare the result with a control group’s response to a neutral placebo. Then this ‘fake treatment’ will confirm, or question its effectiveness.
A Major Milestone for AstraZeneca Researchers
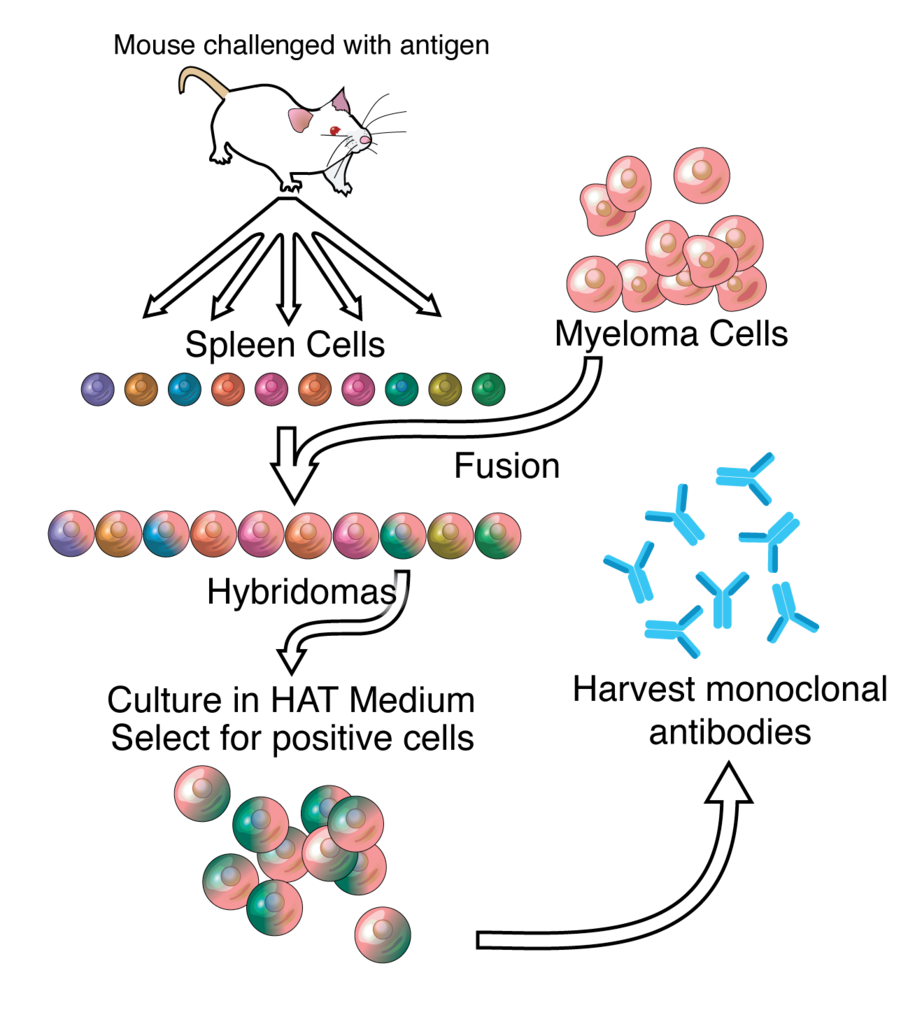
Contagion Live explains AZD7442 comprises two monoclonal antibodies obtained from infected COVID-19 patients. Therefore they come from a unique parent cell ensuring a highly reproducible and scalable, unlimited production source.
Thus the AstraZeneca two antibody vaccine trial is an important milestone in the company’s program. The first trial will involve 48 healthy people aged 18 to 55, and stay in touch for a year after the test. In addition, laboratory synthesis of monoclonal antibodies mimics what happens naturally in nature according to Contagion Live.
The AZD7442 compound is the brainchild of the Vanderbilt University Medical Center that licensed it to AstraZeneca in June 2020. Funding for the trial comes from Defense Advanced Research Projects Agency, and Biomedical Advanced Research and Development Authority. With these impressive credentials we look forward to a successful conclusion.
Related
Two Billion Doses of an Adenovirus Vaccine
Virus Could End in Two Years Says WHO
Preview Image: Monoclonal Antibody Synthesis